Articular cartilage grafting
Articular cartilage is the smooth white shiny layer of tissue that is a few millimetres thick and that covers the surfaces of the bones within the knee joint. The function of the articular cartilage is to make the bone surfaces within the joint smooth and low-friction, so that the knee joint can bend and straighten literally millions of times a year with normally only very little, if any, wear and tear.
Articular cartilage is an amazing tissue, because it has the lowest coefficient of friction of any substance known to man (i.e. it is extremely slippery). However, it has no actual blood supply, and the cells within the cartilage are relatively sparse and not very metabolically active, and they get their oxygen and their nutrition simply from diffusion from the synovial joint fluid. This means that articular cartilage has very little, if any, ability to repair itself if it does become damaged, and it cannot grow back if it wears away. Therefore, articular cartilage damage is something that normally tends to be progressive, and it tends to get worse with time, not better.
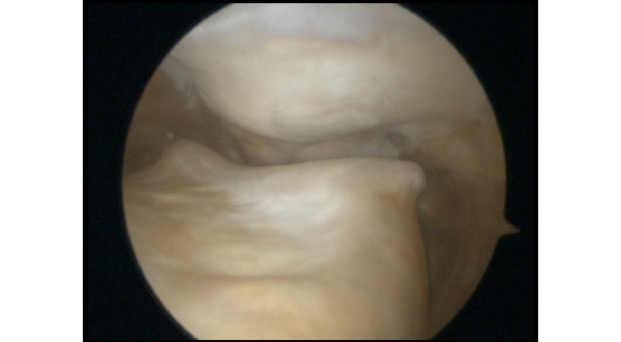
If you have widespread ‘wear and tear’ in a knee joint, with widespread thinning or complete loss of the articular cartilage (with bare bone exposed), then our ability to surgically replace the missing articular cartilage is extremely limited. However, if you have a small(ish) focal patch of discrete articular cartilage damage/loss, then then it is possible to perform articular cartilage grafting, which is a reasonably effective way of replacing the missing tissue and reducing a patient’s symptoms, improving their function, helping them keep their knee going for longer, and either avoiding or at least delaying potential subsequent joint replacement surgery.
What DOESN'T work in degenerate knees!
There are NO kinds of joint injection that can make articular cartilage grow back in a knee, and that includes steroid injections, hyaluronic acid injections, PRP and ‘stem cells’. In particular, be especially wary of any dodgy ‘stem cell’ clinics and their completely false marketing promises. CLICK HERE to read about the dangers of the ‘stem cell’ salesmen!
Likewise, there are many different supplements available and some people absolutely swear by them – but none of them actually prevent or reverse arthritis or make cartilage grow back.
One surgical procedure to be particularly wary of is microfacture – this is a procedure performed via keyhole surgery (knee arthroscopy), and it involves drilling multiple small holes in the surface of the bone at the base of a full-thickness articular cartilage defect. This allows the bone surface to bleed, and the blood from the subchondral bone is rich in bone marrow and contains some stem cells – this forms a clot, and the clot matures into a layer of fibrocartilage, which is half way between normal cartilage and scar tissue. Microfracture is quite effective for small focal cartilage defects that are <2cm2 (i.e. 1.4cm x 1.4cm, which is actually pretty small) – however, it doesn’t tend to give good outcomes for cartilage defects larger than this. Also, it only tends to work well with discrete focal defects where the surround cartilage is in good condition – but it doesn’t work well for more generalised ‘wear and tear’ where there is generalised thinning of the surrounding cartilage.
Microfracture is a reasonably good treatment option for isolated small focal full-thickness articular cartilage defects, but it is not suitable for large defects or in knees where the articular cartilage is simply wearing thin and/or where the damage is more severe and/or widespread. Therefore, microfracture is NOT an appropriate treatment for arthritic knees.
ACI / MACI
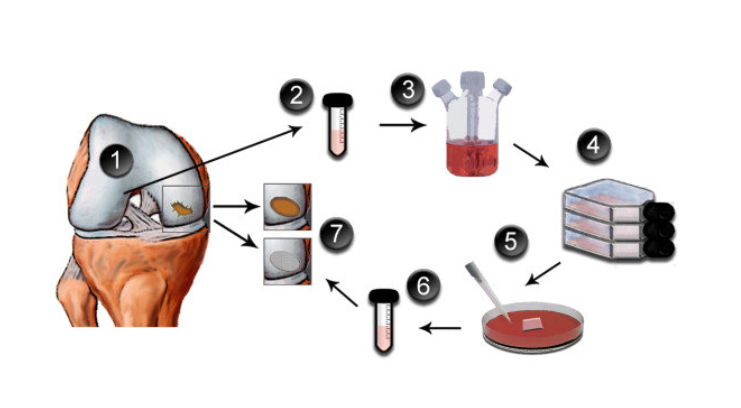
ACI = autologous chondrocyte implantation. It is a two-stage procedure: the first stage involves harvesting some cartilage tissue from the edges of the joint surfaces via arthroscopy; the cartilage cells are then cultured in a lab for 6 weeks; the second-stage surgery involves implanting the cultured cells back into the knee.
ACI was first performed in humans all the way back in 1987, and the first case series was reported by Brittberg in 1994 [CLICK HERE for the reference]. Since then, the technique of ACI has evolved…
The first version of ACI involved the use of a patch of periosteum (the fibrous layer covering the non-articular surface of a long bone), with the cells being injected under the patch in the form of a liquid / gel. However, with this technique there was an appropriately 25% early re-operation rate for issues such as overgrowth of tissue.
The second version of ACI involved using a collagen membrane (obtained from porcine (pig) tissue) instead of periosteum, and this reduced the re-operation date.
The third version of ACI involved pre-seeding the cultured cartilage cells onto a collagen membrane, so that a ‘living’ membrane was implanted into the joint. This is referred to as MACI.
Unfortunately, despite increasing high-quality evidence, for many years the National Institute for Clinical Excellence (NICE) refused to endorse ACI / MACI in the UK. As a direct result of this, only very small numbers of procedures were performed. Thankfully, however, in 2017 NICE reassessed ACI, and this time they changed their mind and they decided that there was actually sufficient evidence for them to be able to endorse the procedure of ACI as both effective and cost-efficient, but with some specific caveats:
- the person should not already have had previous surgery to repair articular cartilage defect,
- there must be minimal osteoarthritic damage to the knee,
- the defect should be over 2cm2 and
- the surgery should only be performed by a specialist surgeon working at a specialist centre.
CLICK HERE to read the 2017 NICE ACI appraisal.
By the time that NICE had backtracked and changed their mind, unfortunately the companies providing the cell culture found that because of the very small numbers, the process was simply not commercially viable. Therefore ACI / MACI ended up becoming unavailable in the UK except in one or two small centres, such as in Oswestry in North Wales, where they had their own cell-growing lab. Sadly, ‘traditional’ MACI is no longer readily available in the UK.
More recently, however, a German company called Codon have developed ‘Spherox’, which is a form of ACI that produces multiple small spheres of cultured cartilage cells, called ‘chondrospheres’. This was assessed by NICE and deemed equivalent to ACI, and hence its use in the UK has now been approved:
CLICK HERE to read the 2018 NICE Technology Appraisal on Spherox.
All of these various ACI-like procedures are broadly similar, in that they all involve:
- A first procedure to harvest some of the patient’s own cartilage cells (via keyhole surgery).
- A 6-week period of cell culture in a lab.
- A second procedure to surgically implant the cartilage graft (via open surgery).
- They are complicated and ‘fiddly’ (i.e. there is plenty that can go wrong!).
- They involve 2-stage surgery (i.e. 2 operations, not just 1)
- They are very expensive – the process of cell culturing alone (without the cost of the actual operations themselves) costs at least £10,000, meaning that the overall total cost is more in the region of about £20,000 or so per case.
In terms of actual clinical outcomes, when ACI/MACI is used appropriately (i.e. for the right cartilage defect, in the correct knee, in a suitable patient and at the right time), then it can be associated with 80% success rates when viewed at 5-year follow-up.
Most importantly, however, ACI / MACI / Spherox are procedure that are specifically intended for focal cartilage defects in younger patients who do not actually have fully-blown widespread arthritis in their knee, and unfortunately this kind of surgery is not suitable for the large majority of patients with early arthritis.
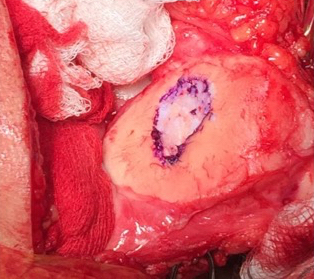
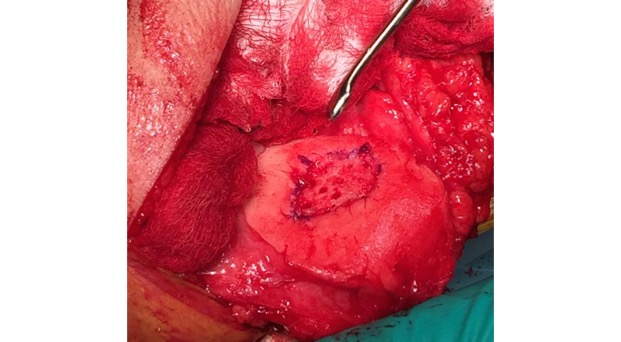
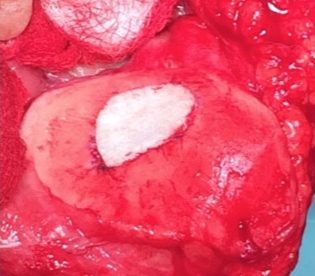
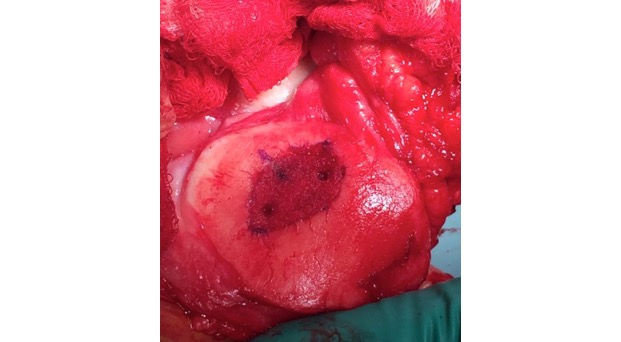
Single-stage grafting with AMIC
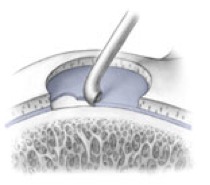
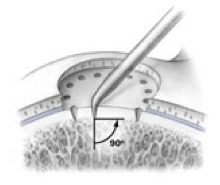
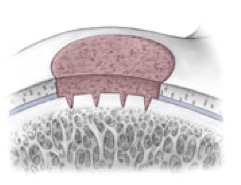
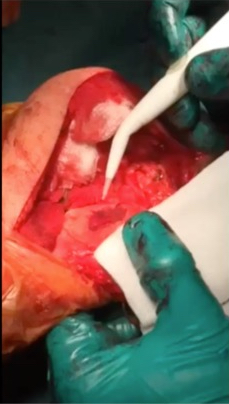
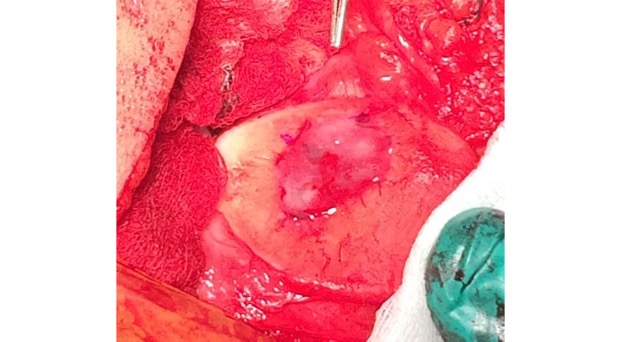
AMIC = Autologous Membrane Induced Chondrogenesis, and it evolved in response to the relate lack of availability of ACI/MACI in many centres. AMIC involves ‘nanofracture’ (drilling very narrow (0.9mm) diameter holes) in the subchondral bone plate (the bare bone at the base of a full-thickness cartilage defect), and then covering the defect with a bioabsorbable patch. The holes in the surface of the bone cause bleeding, and (as with microfracture) the blood contains cells from the bone marrow that include mesenchymal stem cells, with the patch covering the area and keeping the cells in place whilst the area heals.
There are some very important differences between microfracture and AMIC:
- Microfracture is only appropriate for small cartilage defects; however, AMIC can be used for much larger defects of several cm2.
- The new tissue that forms from microfracture is ‘fibrocartilage, which is half way between normal cartilage tissue and scar tissue, but it is not ‘normal’ cartilage tissue. The new tissue that forms from AMIC has been shown to be hyaline-like cartilage (i.e. much closer to normal cartilage tissue).
- With microfracture, the holes in the surface of the bone are larger, and this can often lead to subchondral osteophyte formation. With AMIC, however, the holes are much smaller and as a result there is a much lower incidence of excessive bone growth and osteophyte formation.
The clinical outcomes of AMIC appear to be very similar indeed to those seen from ACI / MACI. However, AMIC offers a number of potential significant advantages over ACI / MACI:
- ACI / MACI is a two-stage procedure: i.e. it requires two separate operations. AMIC, however, is a single-stage procedure, and everything can be done with just one single operation.
- ACI / MACI is extremely troublesome, in terms of logistical and practical organisation, as the living harvested cells have to be sent in a very carefully controlled fashion to the lab, where they are then cultured. The culturing is a complex process, and at the end of this the living cells have to be transported carefully to the relevant hospital and implanted as close to 6 weeks later as possible. The more complex a series of procedures like this is, the more there is to potentially go wrong!
- ACI / MACI is very expensive. Just the culturing of the cells alone can cost in excess of £10,000, and by the time you add onto that the cost of 2 operations, the overall cost can easily exceed £20,000. By comparison, most of the scaffolds used for AMIC-type procedures are relatively cheap, and tend to cost about £1000 to £2000, and the total cost of an AMIC procedure tends to be under £10,000, which is less than half the cost of ACI/MACI.
For the above reasons, an AMIC-type technique has been my personal procedure of preference for articular cartilage grafting in the knee, and I have been performing this kind of surgery now since 2012.
Importantly, just like ACI / MACI, AMIC is not a suitable for treatment for patients with widespread / fully-blown arthritis of the knee.
Chondrotissue
The scaffold that I have tended to use for my AMIC-type cases is called ‘Chondrotissue’. This is a scaffold of woven polyglycolic acid fibres impregnated with hyaluronic acid, and it looks like ‘fuzzy felt’. The scaffold is fully biocompatible (it doesn’t cause any adverse reactions) and it is bioabsorbable (meaning that the scaffold gradually absorbs as the new cartilage tissue forms underneath.
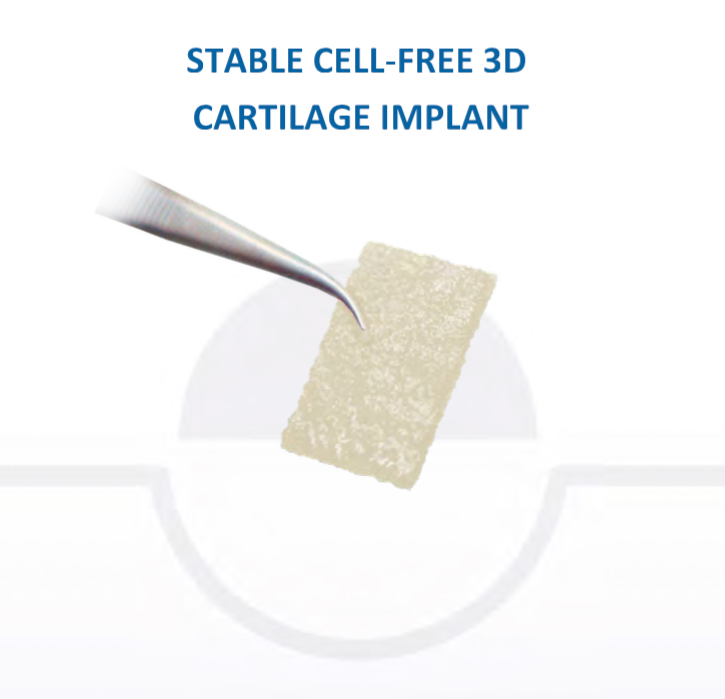
CLICK HERE to read more about Chondrotissue.
In addition, since 2014 I have also been using Vivostat PRF as an integral part of the cartilage grafting procedure. Vivostat is an autologous (it comes from the patient’s own blood), bioabsorbable (it dissolves with time), bioactive (it releases growth factors over the first few days, which promotes healing) biological glue. The sticky part of the ‘glue’ comes from the patient’s own fibrin (which is part of what forms normal blood clots) and the bioactive part comes from a high concentration of platelets (which again come from the patient’s own blood, and it is these that release the growth factors).

CLICK HERE to read more about Vivostat PRF biological glue.